Relationship Between Celsius and Kelvin: A Simple Guide
When looking at a standard thermometer in most parts of […]
When looking at a standard thermometer in most parts of the world, the numbers displayed are usually in Celsius. This scale is the standard for almost every daily activity. People rely on Celsius to decide if a coat is necessary for the weather or to check if an oven is hot enough to bake a cake.
However, a different symbol often appears in science textbooks and laboratories: a capital K. This letter stands for Kelvin.
For students or anyone interested in science, seeing two different systems can be confusing. Fortunately, the relationship between Celsius and Kelvin is straightforward. It does not require advanced calculus or complicated formulas. The connection is nothing more than a simple shift in numbers.
What Is the Relationship Between Celsius and Kelvin?
The most critical fact to understand is that Celsius and Kelvin use the exact same ruler size.
To visualize this, imagine two different ladders standing side by side. The steps on the Celsius ladder are spaced exactly the same distance apart as the steps on the Kelvin ladder. Climbing one step up on the Celsius scale represents the same increase in heat as climbing one step up on the Kelvin scale.
- Equal Magnitude: A temperature rise of 1 degree Celsius () is exactly equal to a rise of 1 Kelvin ().
- No Stretching: Unlike Fahrenheit, which uses smaller steps, these two scales move in perfect sync.
The only real difference lies in where each scale decides to place the label “zero.”
The Magic Number: 273.15
Because the starting lines are different, a specific number bridges the gap: 273.15.
This number is the key to the relationship between Celsius and Kelvin. To switch from one system to the other, one simply needs to add or subtract this specific amount. There is no need for multiplication or division, making it much easier than converting to Fahrenheit.
Understanding the Starting Points
To fully grasp the relationship between Celsius and Kelvin, it helps to look at what “Zero” represents for each system. They were designed with different goals in mind.
Celsius: Based on Water
The Celsius scale was created with human life and Earth’s environment in mind. Since water is essential for life, the scale uses water as its anchor points.
- 0°C (Freezing Point): This is the temperature where liquid water turns into solid ice.
- 100°C (Boiling Point): This is the temperature where liquid water turns into gas (steam).
Because it aligns with the phases of water, Celsius is incredibly useful for describing the weather or cooking food.
Kelvin: Based on Energy
The Kelvin scale is designed specifically for the needs of scientists, physicists, and astronomers. They needed a scale that begins at the true “bottom” of the universe’s temperature range.
- 0 K (Absolute Zero): This represents the coldest possible temperature in existence. At this point, all heat energy vanishes, and atoms effectively stop moving.
- No Negatives: Since 0 K is the absolute floor, the Kelvin scale contains no negative numbers. This makes calculations in physics much cleaner.
How to Convert Between Celsius and Kelvin
Since the relationship between Celsius and Kelvin is defined by a simple shift, performing conversions is a quick process.
Converting Celsius to Kelvin
When a temperature is known in Celsius, finding the equivalent in Kelvin requires a simple addition. The formula shifts the scale up by the magic number.
The Formula:
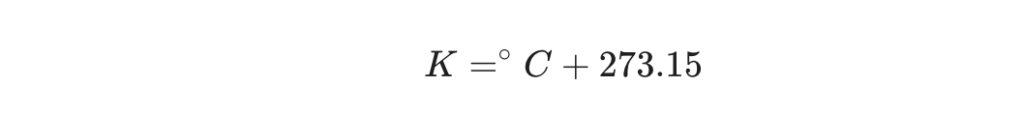
Example Scenario:
Water boils at . To express this in Kelvin:
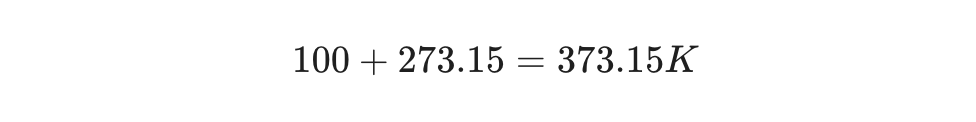
Converting Kelvin to Celsius
Conversely, if a scientist provides a temperature in Kelvin, finding the relatable Celsius number requires subtraction.
The Formula:
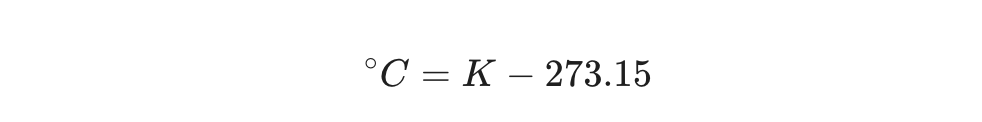
Example Scenario:
Absolute zero is . To express this in Celsius:
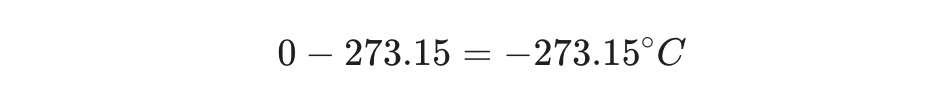
Comparison Table: Celsius vs. Kelvin
The following table demonstrates how common temperature events compare across both scales. It highlights the consistent relationship between Celsius and Kelvin.
| Event | Celsius () | Kelvin () |
|---|---|---|
| Absolute Zero | -273.15 | 0 |
| Water Freezes | 0 | 273.15 |
| Comfortable Room Temp | 20 | 293.15 |
| Human Body Temp | 37 | 310.15 |
| Water Boils | 100 | 373.15 |
Why Do We Use Two Different Scales?
It is natural to wonder why the world does not settle on just one single system. The answer lies in the different applications for each scale.
Celsius is the champion of daily life.
- It is intuitive for the general public.
- People instantly know that is icy and is a hot day.
- The numbers are small and easy to handle for weather reports and thermostats.
Kelvin is the champion of science and math.
- It is essential for equations involving gas laws and thermodynamics.
- Using negative numbers (like ) in complex formulas can lead to mathematical errors or impossible results (like calculating negative volume).
- Kelvin solves this problem by starting at zero and only going up, keeping all values positive.
FAQ
Is 1 degree Celsius the same size as 1 Kelvin?
Yes. The magnitude of the unit is identical. An increase in temperature of 1 degree Celsius represents exactly the same amount of heat energy change as an increase of 1 Kelvin. The steps on the ladder are the same size; the ladders just stand on different ground levels.
Do I use the degree symbol (°) for Kelvin?
No. The correct terminology is “degrees Celsius” (), but for the scientific scale, it is simply “Kelvin.” The symbol is the letter K without a circle. For instance, one writes , never .
Why is 273.15 the conversion number?
This specific number represents the precise offset between absolute zero and the freezing point of water. Since absolute zero occurs at , the Kelvin scale must be shifted by exactly that amount to make absolute zero equal to .
Can Kelvin ever be negative?
No. By scientific definition, is the lowest possible temperature in the physical universe. It is impossible to get colder than “zero movement” of atoms. Therefore, a negative Kelvin temperature does not exist in standard thermodynamics.